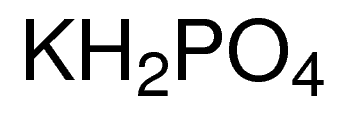A Process for removing toxic heavy metals to produce the high purity NH4H2PO4 and KH2PO4 from a crude phosphoric acid - ScienceDirect
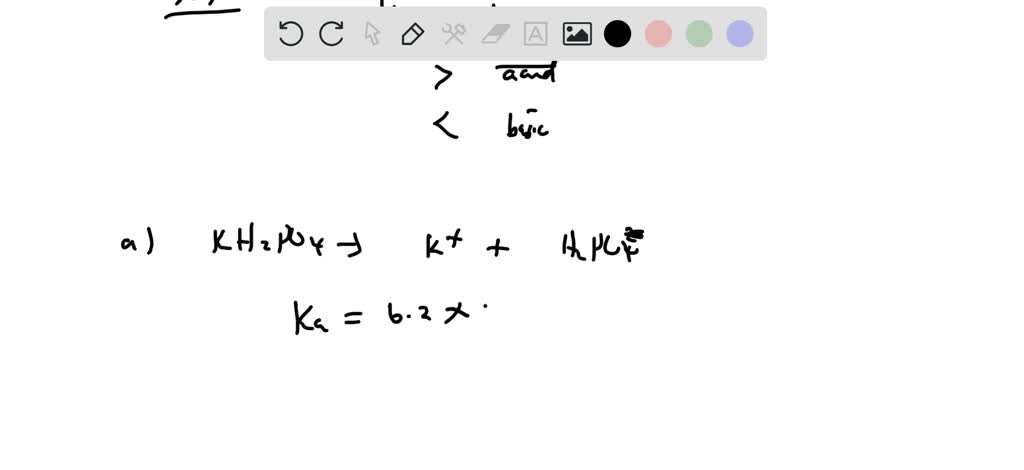
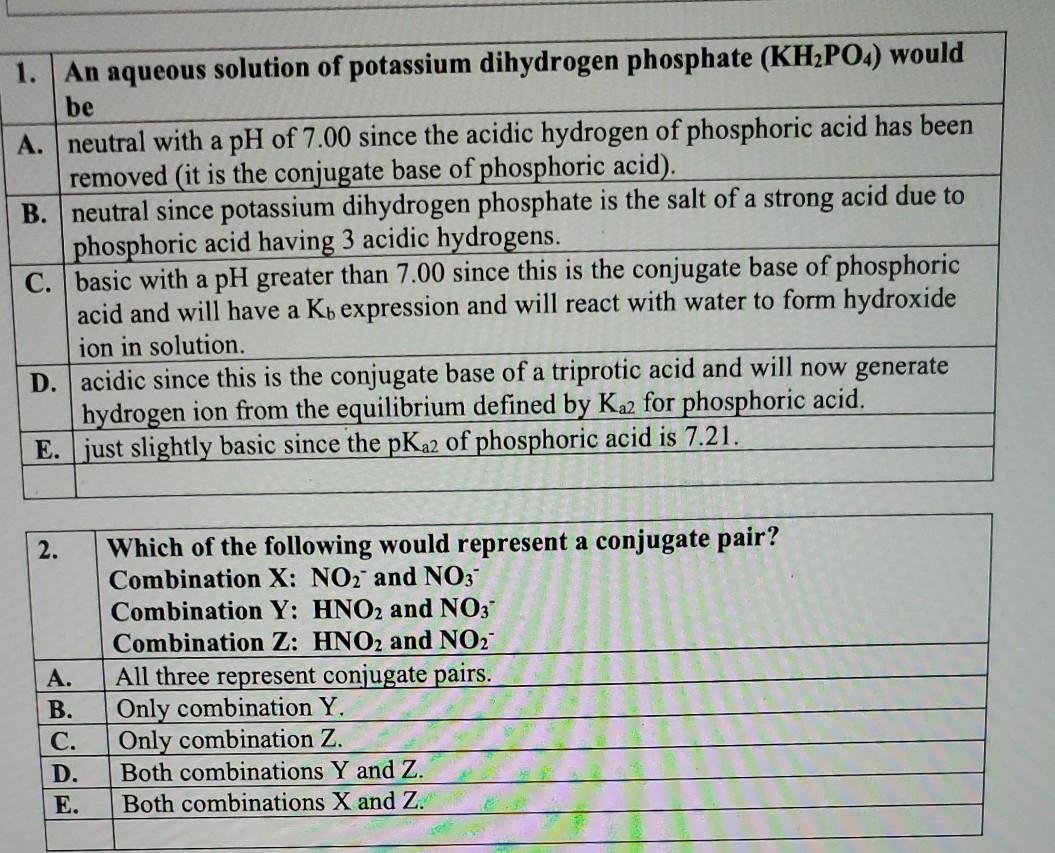
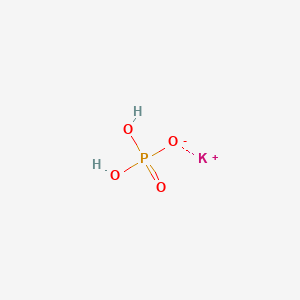
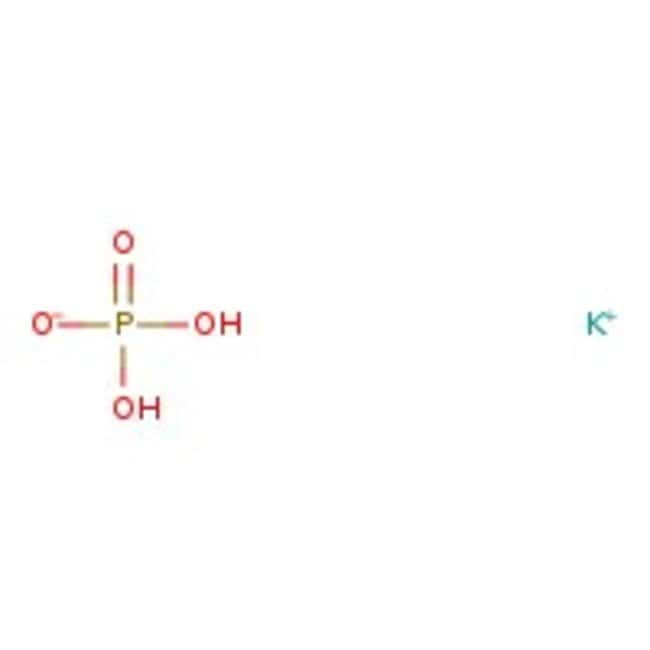
SOLVED:State whether each of the following solutions is acidic, basic, or neutral. (a) potassium dihydrogen phosphate (b) potassium hydrogen carbonate
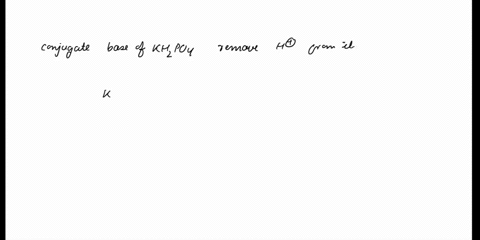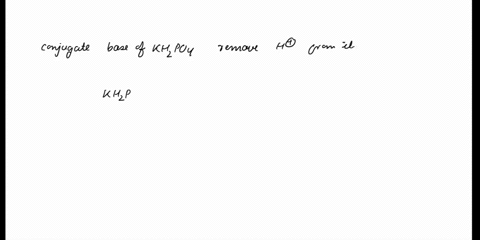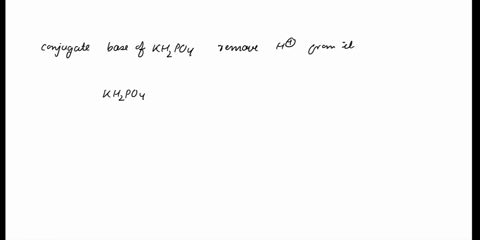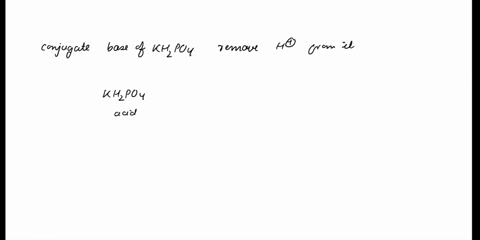
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?
![Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/KH2PO4%20500G.jpg)
Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com





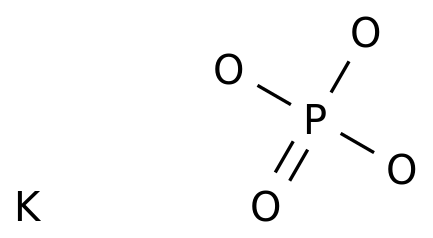
(358).jpg)