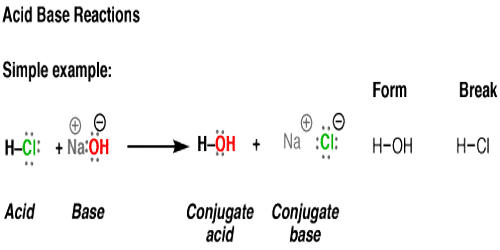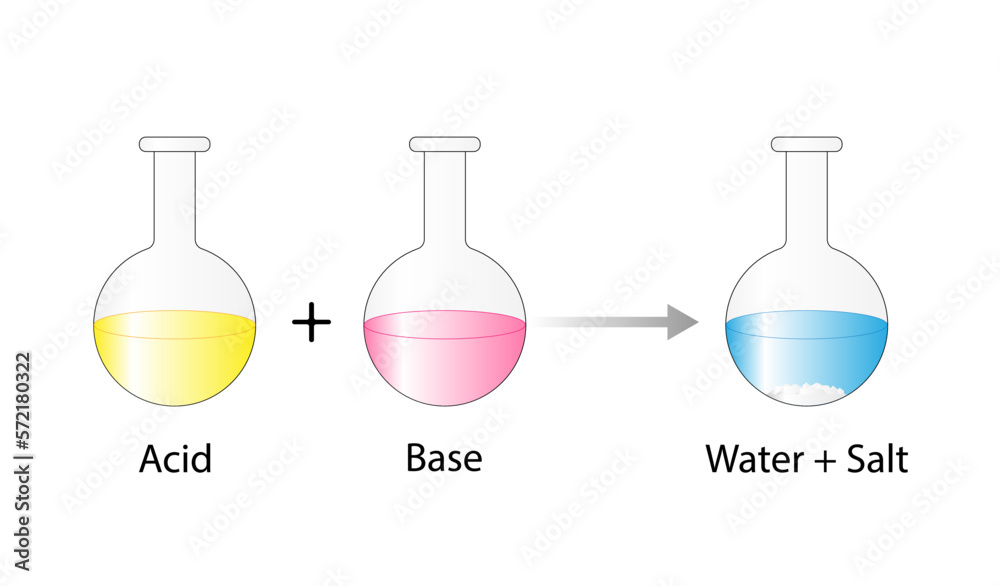
Acid–base reaction. chemical reaction neutralization. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Vector illustration. Stock Vector | Adobe Stock
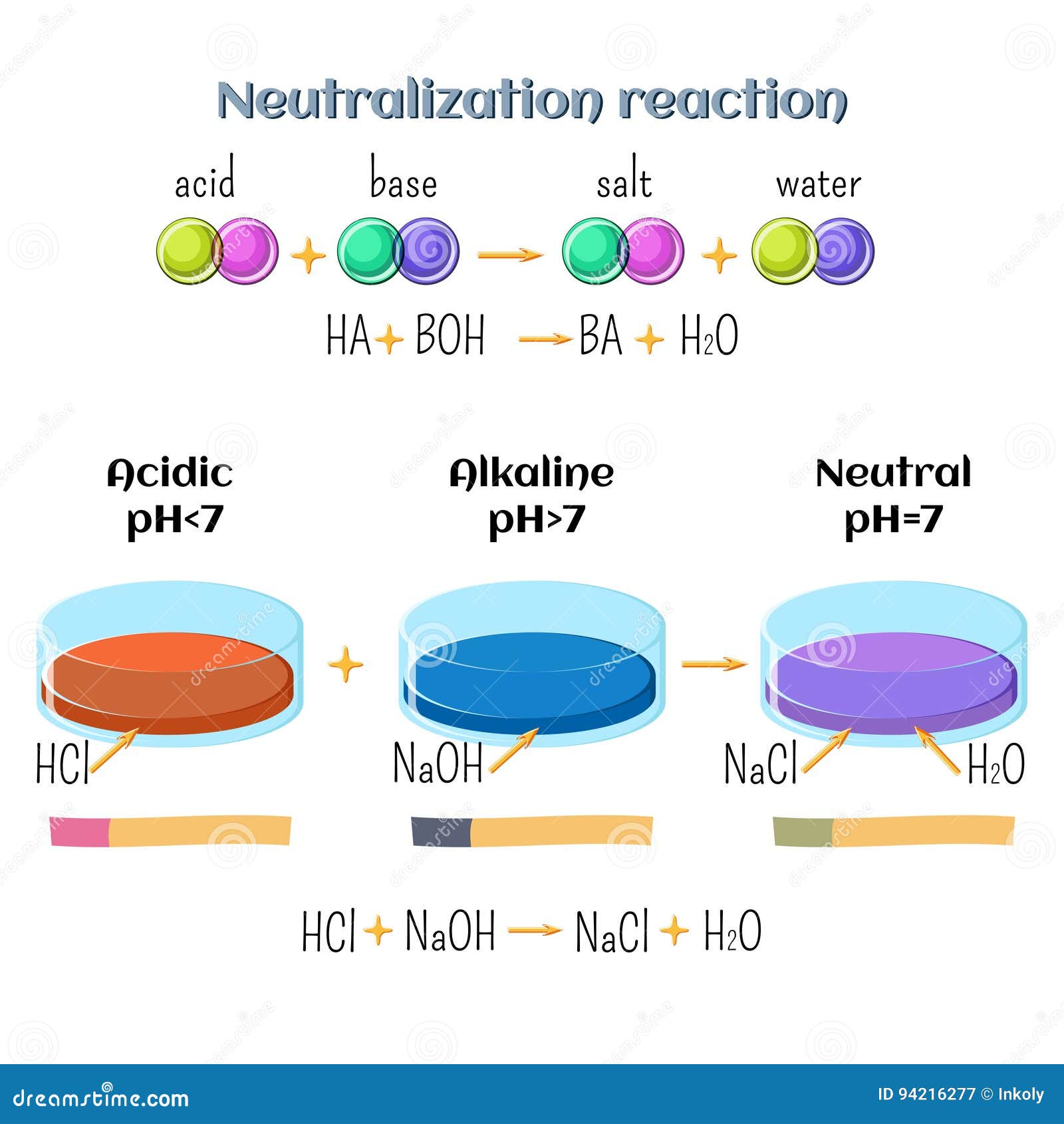
Acid-base, Neutralization Reaction of Hydrochloric Acid and Sodium Hydroxide. Types of Chemical Reactions, Part 6 of 7 Stock Vector - Illustration of acid, atom: 94216277
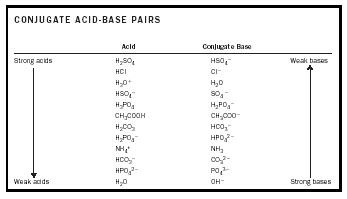
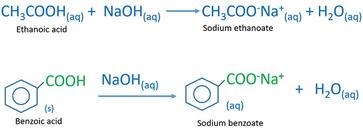
Acid-Base Chemistry - Chemistry Encyclopedia - reaction, water, metal, gas, number, equation, salt, property
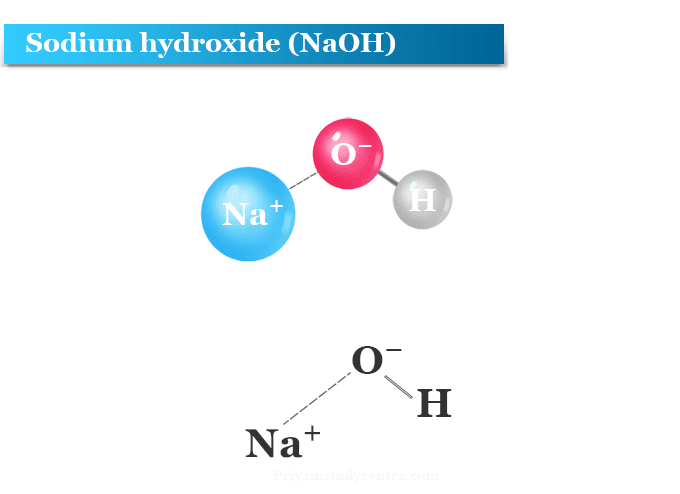
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution? | Socratic