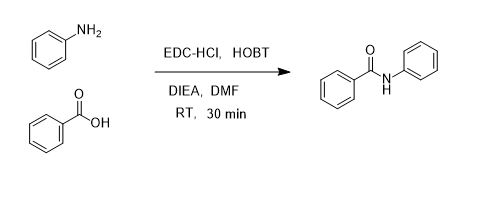
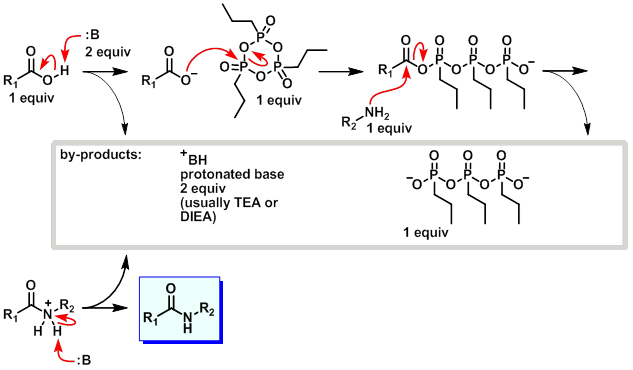
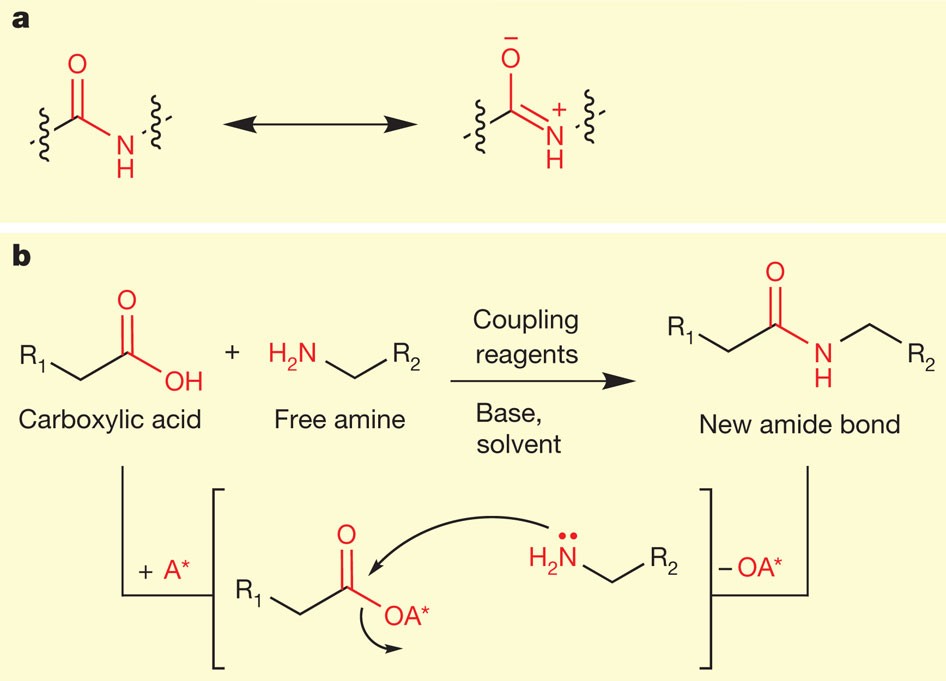
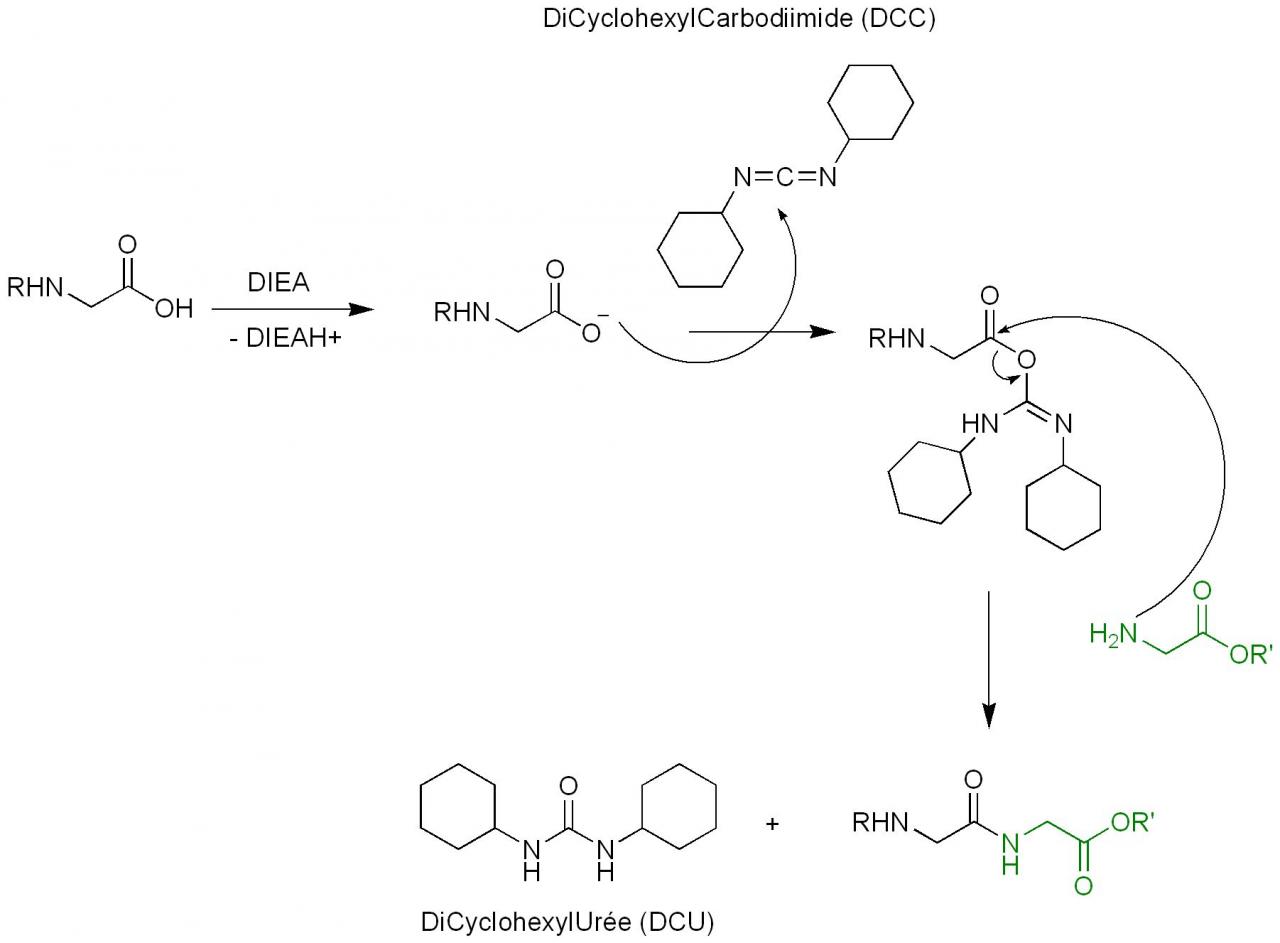
Hydrodehalogenation of Alkyl Iodides with Base-Mediated Hydrogenation and Catalytic Transfer Hydrogenation: Application to the Asymmetric Synthesis of N-Protected α-Methylamines | The Journal of Organic Chemistry

Dual Lewis Acid−Lewis Base Activation in Enantioselective Cyanation of Aldehydes Using Acetyl Cyanide and Cyanoformate as Cyanide Sources | Journal of the American Chemical Society
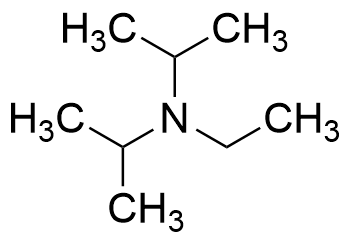
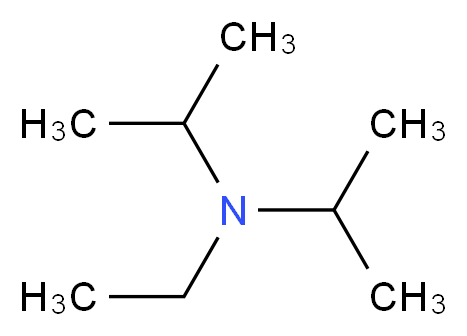
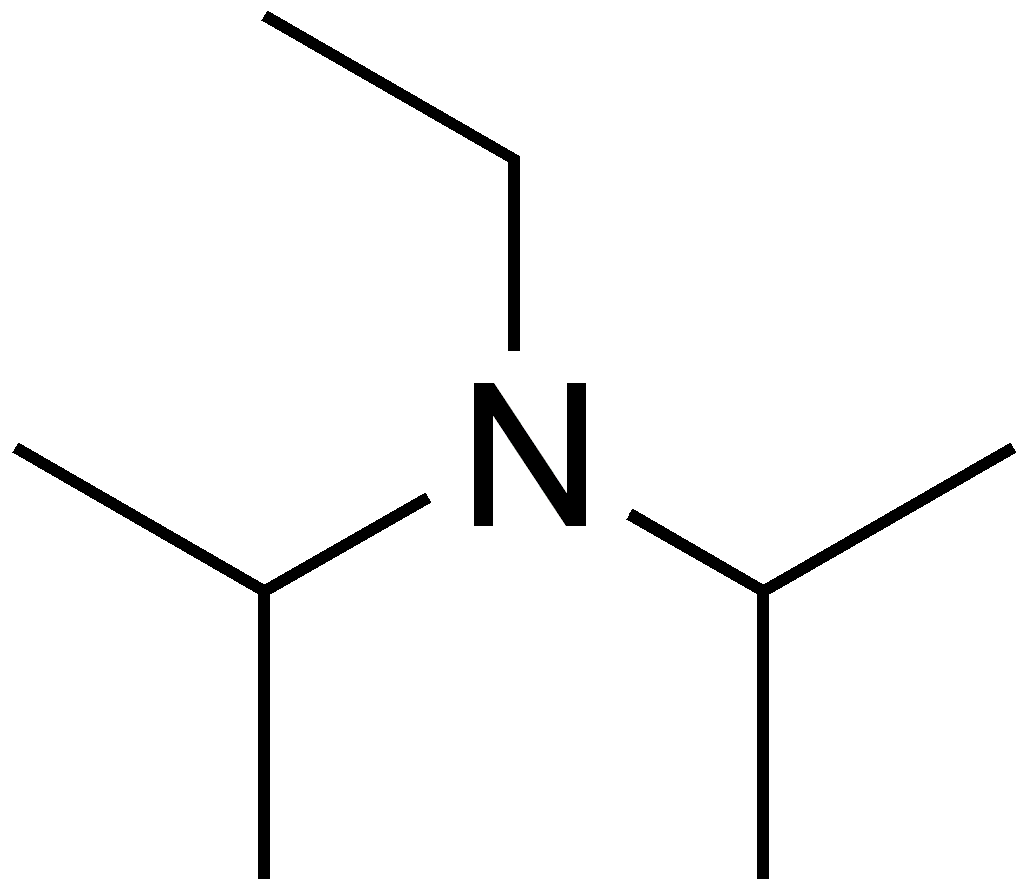
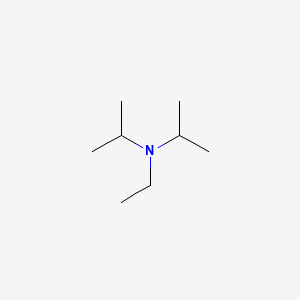
7087-68-5 | N,N-Diisopropylethylamine | 1,1'-Dimethyltriethylamine; Bis(1-methylethyl)ethylamine; DIEA; DIPEA; Diisopropylethylamine; Ethyl-N,N-diisopropylamine; Ethyldiisopropylamine; Huenig's base; Hunig's base; Hunig's reagent; N,N-Bis(1-methylethyl ...

Metal and carbene organocatalytic relay activation of alkynes for stereoselective reactions | Nature Communications

N,N-Diisopropylethylamine;N-Ethyldiisopropylamine;Huenig's base;DIEA;EDIA;Ethyldiisopropylamine,physical properties,suppliers,CAS,MSDS,structure,Molecular Formula, Molecular Weight ,Solubility,boiling point, melting point