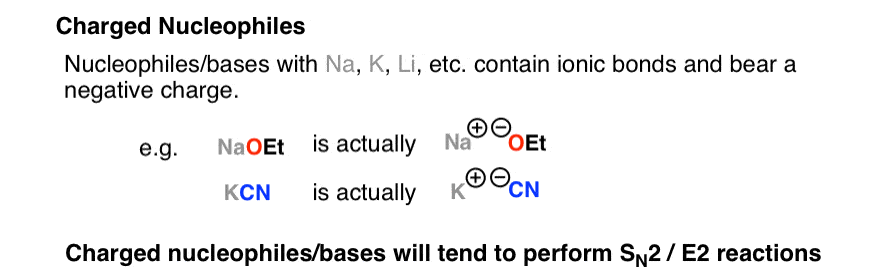
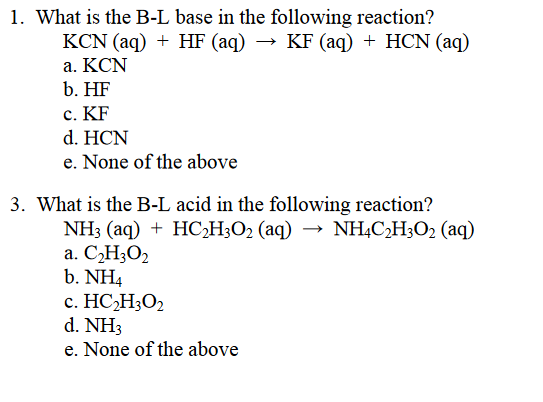
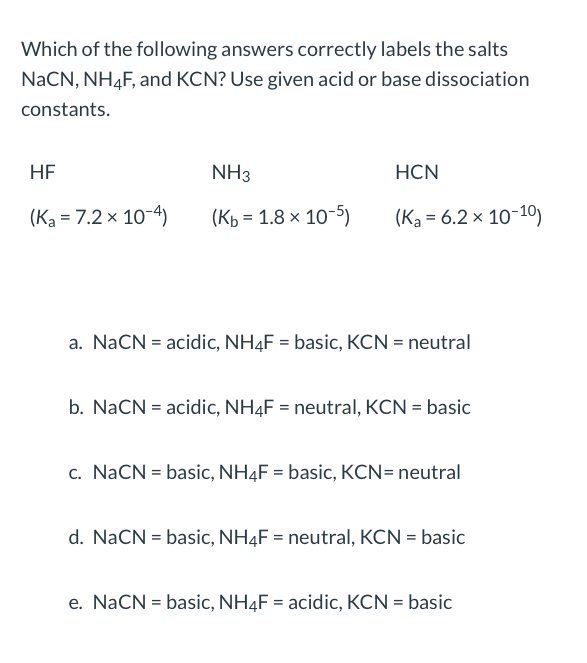
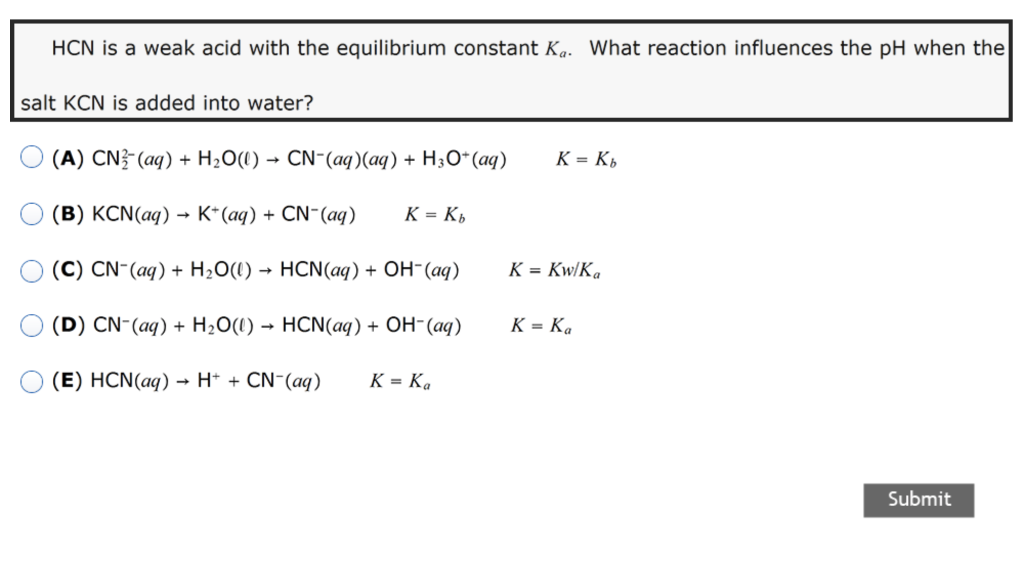
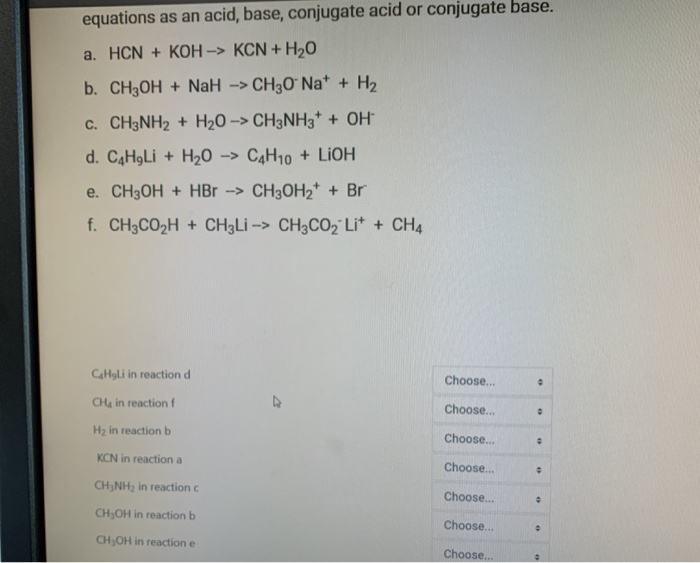
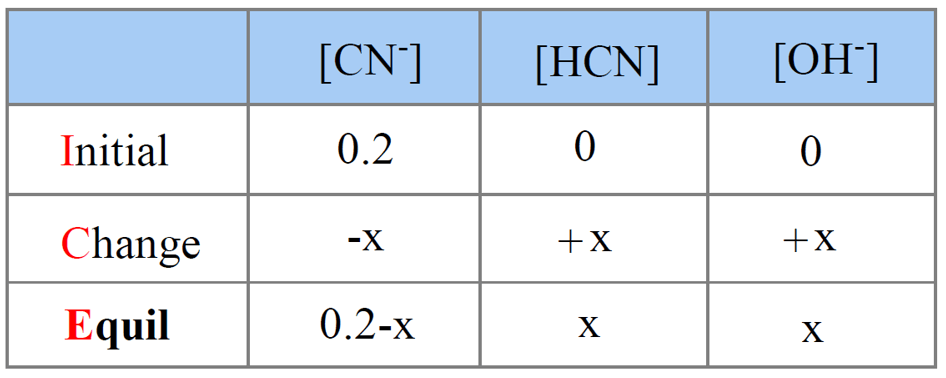
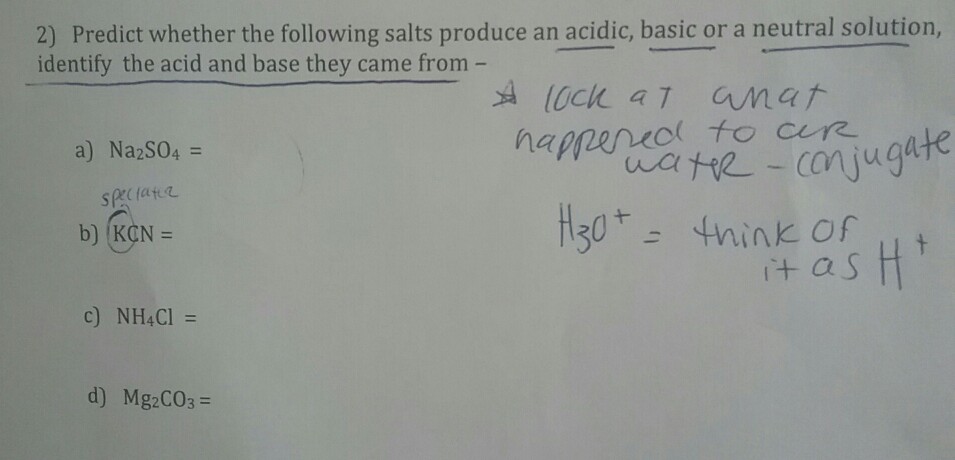SOLVED: You will find it useful to keep in mind that HCN is a weak acid acids: 0.1 mol of NaOH is added to 1.0 L ofa 0.5 MHCN solution. bases: other:
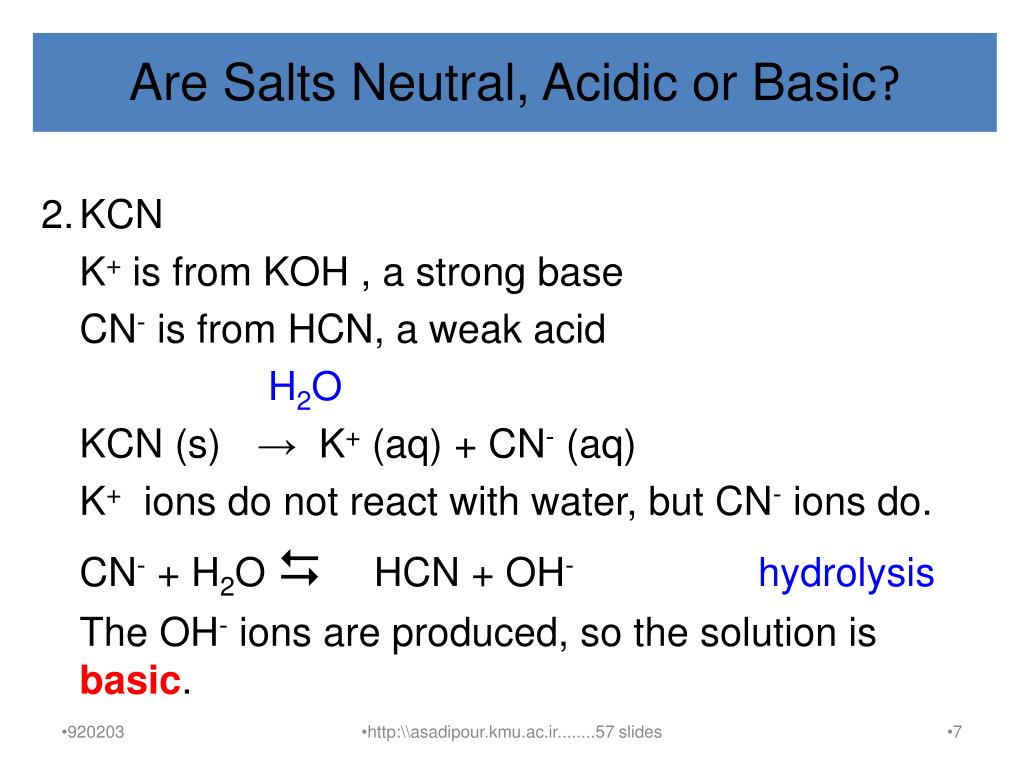
Identify the following salts as neutral acidic or basic.Please include why are they neutral basic or acidic? - Home Work Help - Learn CBSE Forum
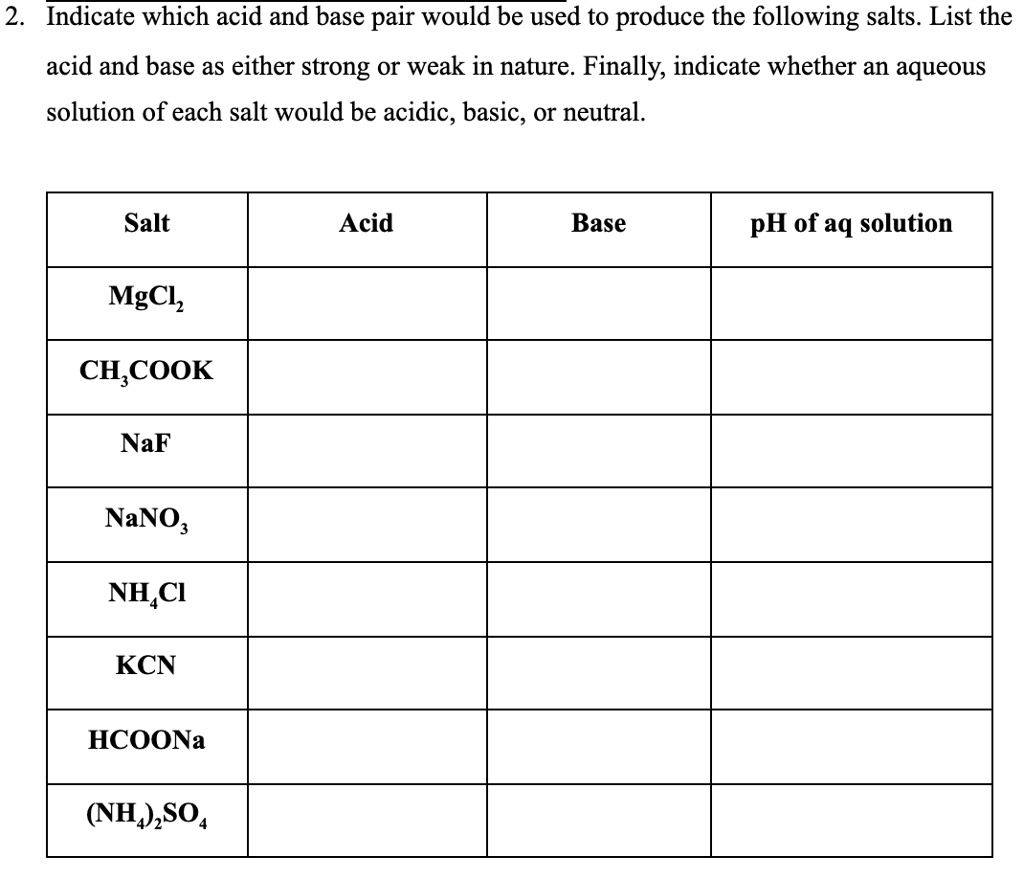
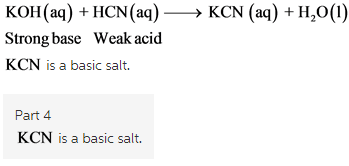
SOLVED: 2 Indicate which acid and base pair would be used to produce the following salts List the acid and base as either strong or weak in nature. Finally, indicate whether an

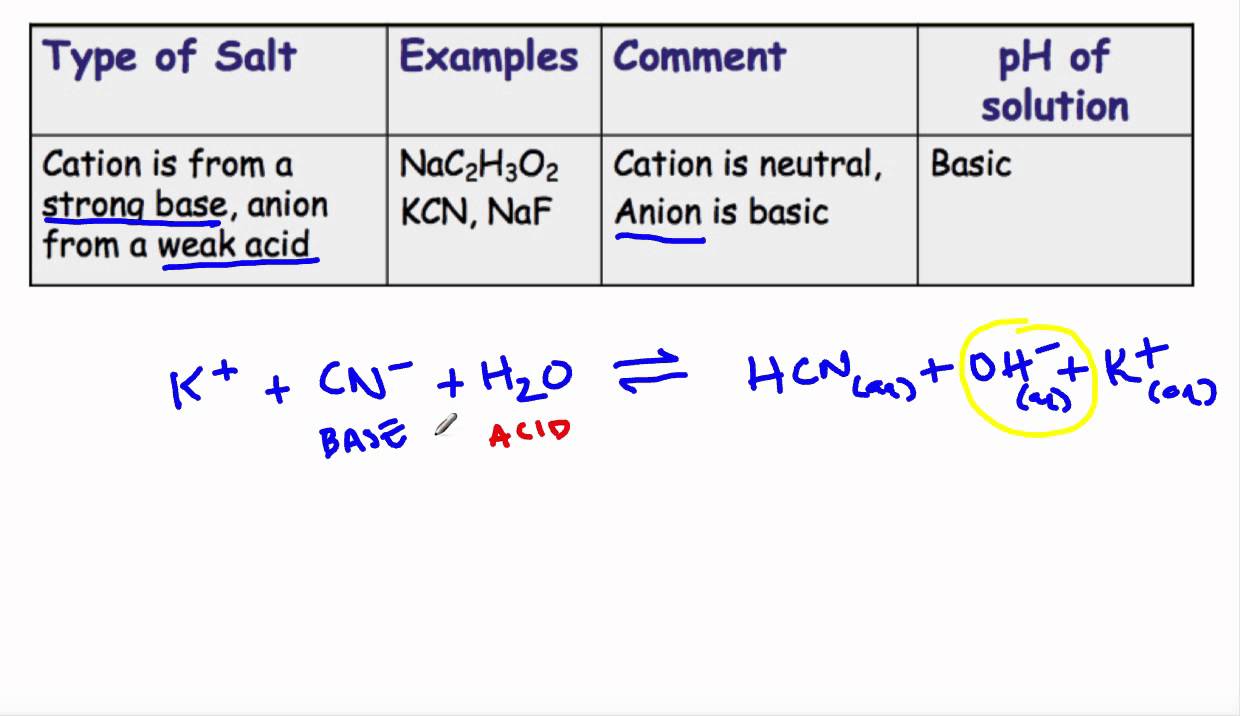
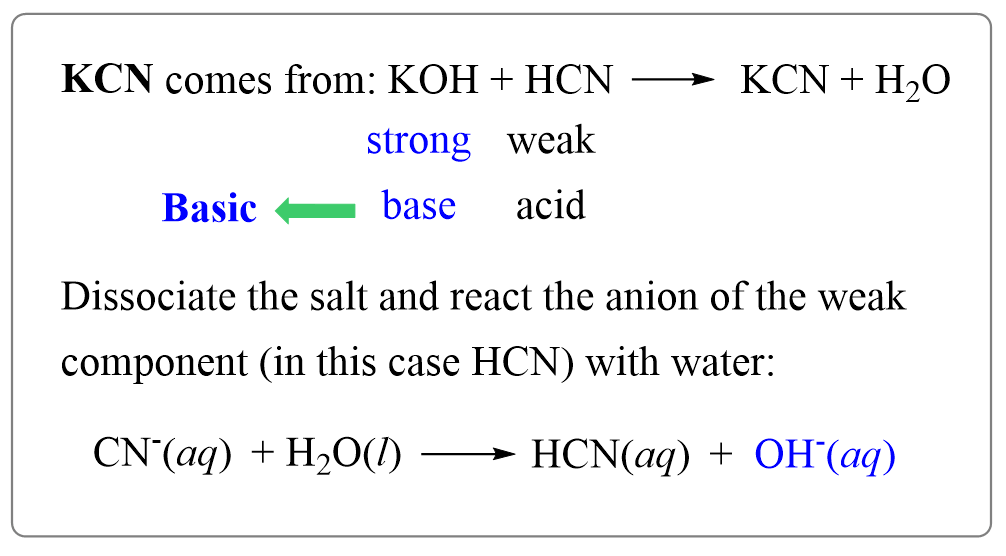
When potassium cyanide reacts with water, will the resulting solution be acidic, alkaline or neutral? Justify your answer.

How to Determine if Salt is Acidic, Basic, or Neutral Example, Problem, Shortcut, Explained Question - YouTube

SOLVED: What are the conjugates of the base KCN and the acid NHat? HCN; NH3 CN , NH3 HzCN+, NHs2+ CN , NHs2+

12) KCN (Potassium Cyanide) 1800's | Organic chemistry, Organic chemistry books, Organic chemistry reactions